Label-free metabolic imaging by mid-infrared optoacoustic microscopy in living cells
- Abstract number
- 421
- Event
- Virtual Early Career European Microscopy Congress 2020
- Presentation Form
- Submitted Oral
- DOI
- 10.22443/rms.emc2020.421
- Corresponding Email
- [email protected]
- Session
- LSA.1 - Label-free life science imaging
- Authors
- Miguel A. Pleitez (2, 5), Asrar Ali Khan (1, 6, 7), Alice Soldà (3, 4), Andriy Chmyrov (3, 4), Josefine Reber (3, 4), Francesca Gasparin (3, 4), Markus R. Seeger (3, 4), Benedikt Schätz (2, 4), Stephan Herzig (1, 6, 7), Marcel Scheideler (1, 6, 7), Vasilis Ntziachristos (2, 4)
- Affiliations
-
1. Institute for Diabetes and Cancer, Helmholtz Zentrum München
2. Institute of Biological and Medical Imaging (IBMI), Helmholtz Zentrum München
3. Institute of Biological and Medical Imaging, Helmholtz Zentrum München
4. Chair of Biological Imaging (CBI) and Center for Translational Cancer Research (TranslaTUM), Technische Universität München
5. Chair of Biological Imaging (CBI), Center for Translational Cancer Research (TranslaTUM), Technische Universität München
6. Joint Heidelberg-IDC Translational Diabetes Program, Heidelberg University Hospital
7. Molecular Metabolic Control, Medical Faculty, Technische Universität München
- Keywords
Label-free imaging, living cells, mid-infrared optoacoustic
- Abstract text
We enabled metabolic imaging in living cells based on label-free observation of carbohydrates, lipids, and proteins, by introducing bond-selective mid-infrared optoacoustic microscopy (MiROM).
The use of label-free imaging and biomolecules spectra detection for monitoring metabolism in living cells and tissue represents an interesting, but still challenging achievement for optical microscopy. Imaging techniques exploiting molecular vibrations, such as Raman scattering or mid-infrared absorption, have already demonstrated label-free high chemical specificity for biomolecules. For instance, Coherent Raman Scattering imaging can detect nucleic acids, lipids and proteins in living cells and excised tissues, but its sensitivity, especially in the fingerprint region, is limited to target concentrations above 1 mM, which are insufficient for live-cell analytical imaging, where µM to nM concentrations need to be determined. On the other hand, conventional mid-infrared modalities with high sensitivity in the fingerprint region can analyze mainly dry tissues and fixed cells due to the strong mid-IR absorption of water and their negative-contrast detection scheme.
We developed a label-free bond-selective new technology based on mid-infrared optoacoustic spectroscopy, termed MiROM (Mid-infraRed Optoacoustic Microscopy), which is able to discriminate between biomolecules inside living cells and tissue. MiROM exploits the specific vibrational transitions of biomolecules for highly efficient optoacoustic generation and detection. It circumvents the limitations of conventional mid-infrared microscopy detecting ultrasounds waves, which are less attenuated by the absorption of tissue and water compared to mid-IR photons. As opposed to other techniques, MiROM is a positive contrast method, which means that the more a sample absorbs, the higher is the detected signal. In this way, it allows deeper penetration compared to systems that work in negative contrast, and it does not require to induce sample perturbation, as thickness constriction, for imaging. MiROM achieves low-micromolar concentration sensitivity with negligible cell photodamage using up to three orders of magnitude less laser power than other vibrational spectroscopy imaging techniques.
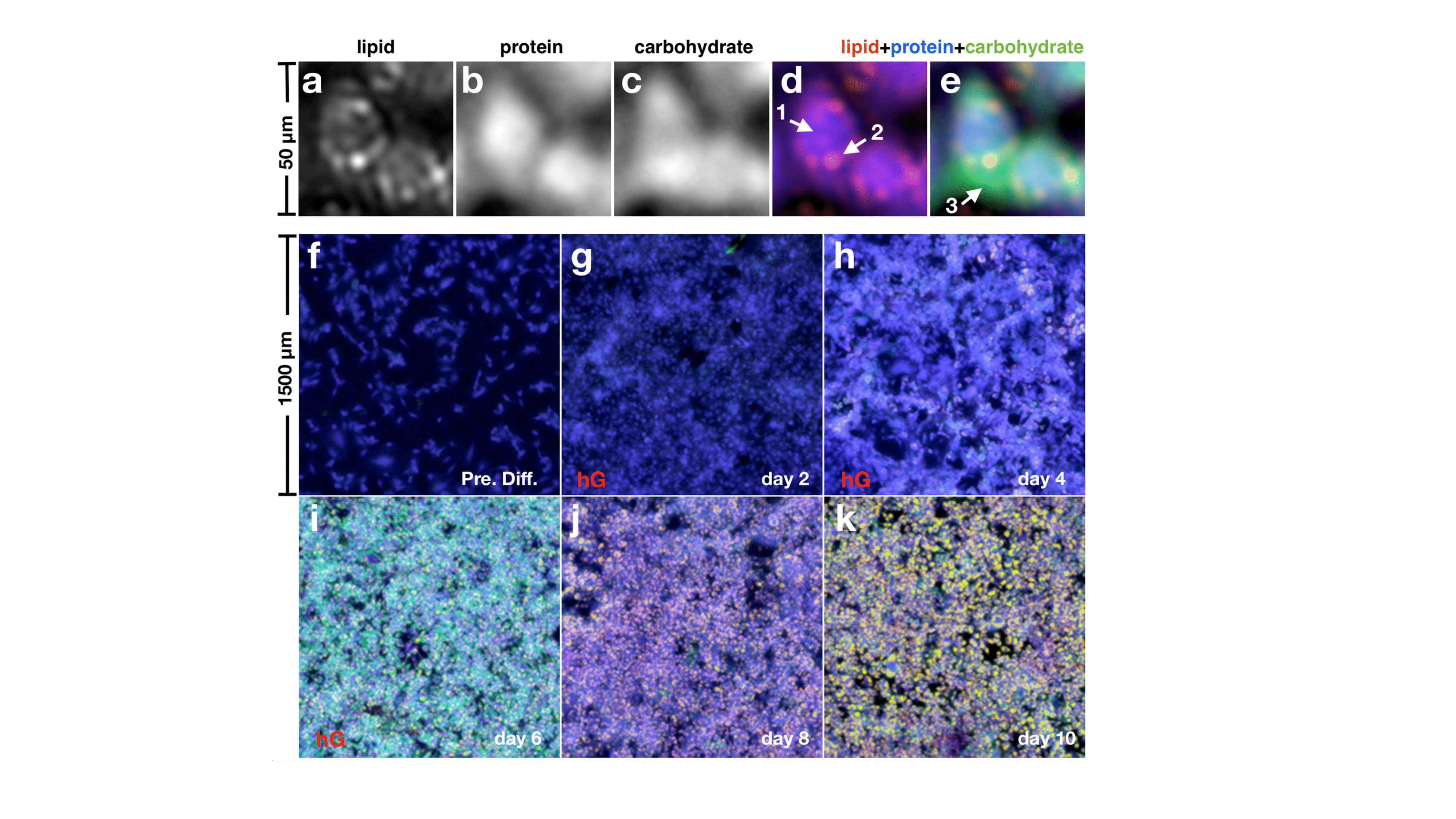
In these studies, we monitored the distribution of biomolecules such as carbohydrates, lipids, and proteins during lipogenesis in 3T3-L1 adipocytes and the dynamic of the isoproterenol-induced lipolysis in brown and white adipocytes, detecting the changes in protein and lipid content. For the first time, we visualized carbohydrate patterns in early-stage adipocytes revealing, over time, an initial spread through the young adipocyte body, followed by a co-localization with lipid droplets upon adipocytes maturation [Figure 1]. We hypothesized that the presence of carbohydrates inside the cell might be related to the capture and accumulation of glucose involved in the biosynthesis of triglycerides to be packed into the lipid-droplets during lipogenesis. This explanation was supported by monitoring lipids, proteins, and carbohydrates in differentiating 3T3-L1 cells, whose uptake of glucose was manipulated changing the concentration of glucose and insulin in the cell medium.
Figure 1.a–e, MiROM micrographs of 3T3-L1 cells at differentiation day 6; a, Lipid map. b, Protein map. c, Carbohydrate map. d, Overlay of lipid (red) and protein (blue) maps. e, Same picture as d, but adding the carbohydrate (green) map. Arrow 1 indicates proteins in the cell body, arrow 2 indicates an LD and arrow 3 indicates an area of carbohydrate accumulation around the growing LDs. f–k, Merged lipid, protein, and carbohydrate maps of 3T3-L1 cells at different incubation days toward LD formation. The overall carbohydrate contrast increases after starting differentiation, and it is broadly distributed in the cells at day 6, around the areas of LD formation, as in e. At days 8 and 10, however, carbohydrate contrast is co-localized with the LDs
Observing lipid and protein dynamics in brown and white adipocytes, we noticed that lipid content slowly increased in both cell lines before starting lipolysis, due to the ongoing lipogenesis. While after isoproterenol-lipolysis induction, lipids content decreased continuously and linearly. As predicted, lipolysis was faster and more extensive in brown adipocytes than in white: in 2 hours lipid content changed up to 30% in brown adipocytes and up to 18% in white. More heterogeneous changes were observed monitoring the protein content, with areas showing an increase and others showing a decrease. This can be explained considering a combination of adipokine secretion, protein degradation and/or protein translation happening in response to lipolysis induction.
To show the capabilities of MiROM to image samples deeper than other mid-IR imaging techniques, we examined the lipid and protein content of a 4 mm slice of freshly excised pancreatic tissue (mouse C57BL/6), where we visualized pancreatic acinar glands with a maximum imaging depth of 90 μm.
The pulsed quantum cascade laser (QCL) was used to induce the optoacoustic signal generation of biomolecules in a broad optical range from 3.4 to 11.0 μm. The detection limit was determined for two reference molecules, DMSO in H2O and albumin in D2O. The acquired MiROM images were processed by a two-pixel Gaussian filtering, outlier removal if necessary, a contrast enhancement to a 0.3% saturation, and histogram normalization. The resolution and signal-to-noise ratio (SNR) of the system were measured from the raw data. For the co-localization analysis of molecules, we applied non-masked and non-thresholded Pearson correlation coefficients to the unprocessed images. The studies of lipogenesis and lipolysis were conducted on 3T3-L1 mouse white preadipocyte and PreBAT cell lines subjected to a differentiation process for 6 days and the carbohydrate content was validated using the Cell Biolabs Total Carbohydrate Assay kit (STA-682, Cell Biolabs) following the manufacturer’s protocol. For the mouse tissue analysis, organs from male C57BL/6J mice were collected and directly placed on the sample holder. All the measurements were performed on a custom-made mid-IR dish with a ZnSe window using carbon tape as spectral reference.
Thanks to its high contrast, image quality, sensitivity, and specificity to endogenous biomolecules in living cells and fresh unprocessed excised tissues, MiROM can become a new analytical tool for observing metabolic processes in real-time, soon applicable also to in vivo studies.
- References
[1] Pleitez, M.A., Khan, A.A., Soldà, A. et al. Nat Biotechnol (2019) doi:10.1038/s41587-019-0359-9
[2] The authors gratefully acknowledge funding from the Deutsche Forschungsgemeinschaft (DFG), Germany (Gottfried Wilhelm Leibniz Prize 2013; NT 3/10-1), as well as from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program under grant agreement No 694968 (PREMSOT).