Label-Free Third Harmonic Generation Imaging of Lipid Droplets in Live Fungus Cells Phycomyces blakesleeanus
- Abstract number
- 1430
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.1430
- Corresponding Email
- [email protected]
- Session
- LSA.1 - Label-free life science imaging
- Authors
- Tanja Pajic (1), Dunja Stefanovic (1), Natasa Todorovic (2), Miroslav Zivic (1), Mihailo D. Rabasovic (3), Aleksandar Krmpot (3)
- Affiliations
-
1. Faculty of Biology, University of Belgrade
2. Institute for Biological Research, University of Belgrade
3. Institute of Physics Belgrade, University of Belgrade
- Keywords
Third Harmonic Generation, Label-free imaging, In Vivo imaging, Nonlinear Laser Scanning Microscopy, fungus Phycomyces blakesleeanus
- Abstract text
The application of Third Harmonic Generation (THG) microscopy on lipid droplet (LD) imaging in filamentous fungus organism Phycomyces blakesleeanus is presented, together with corroboration of the identity of THG bright structures. Physiological perturbation of cell metabolism that is expected to increase the number and size of LDs was undertaken in order to confirm the method can be used for physiological in vivo comparative studies.
Lipid droplets (LDs) are organelles present in most cell types and organisms including yeast and animals and play an important role in cellular metabolism. LDs are dynamic structures that interact with molecules and other organelles involved in energy metabolism. Depending on the cellular metabolic state, the number, size, composition, and distribution of the LDs can be altered [1]. Their increased biogenesis occurs in response to nutrient restriction and oxidative stress [2]. Emerging realization of their importance in normal cell metabolism as well as in the number of pathologies is in contrast with the relatively scarce knowledge about LD physiology. Our aim is to demonstrate that THG imaging technique can be used for in vivo label-free physiological investigation of LDs during cellular metabolic changes.
A suitable method is required for label-free in vivo imaging of lipid droplets because the fixation process destroys or alters their structures. Our choice is the nonlinear laser scanning microscopy technique, i.e. its modalities THG [3] and two-photon fluorescence excitation (TPEF) [4]. THG is a nonlinear coherent optical effect in which the incident laser beam interacts with a medium, producing the light of exactly three times shorter wavelength than the incidental one. THG phenomenon is employed in laser scanning microscopy that utilizes ultrashort laser pulses for imaging. THG mostly occurs at interfaces (e.g. water-lipid) where the change of refractive index is steep. Therefore, cell membranes and lipid droplets are major sources of the THG signal [5].
For in vivo THG imaging of label-free, > 24-hour old hyphae, we used 1040 nm, 200 fs pulses from Yb KGW laser. Detection was performed in the transmission arm by PMT through the Hoya glass UV filter with the peak at 340nm. The fungi were grown in a standard liquid medium and after 24 hours were divided into two groups, centrifuged and the control group was resuspended in standard medium and treatment in the nitrogen-free medium. Before the imaging, the fungi cells were placed between two cover glasses separated by a distancer. Cover glasses were of 0.17 μm thickness in order to match the objective lens requirements and for better transmission of the THG signal. Nile red staining of lipid droplets was used for colocalization experiments. The TPEF of Nile Red dye was excited by the same laser and the signal was collected through the 400-700 nm bandpass filter. The laser beam was focused with the Zeiss Plan Neofluar 40x1.3 objective lens, in both, TPEF and THG imaging.
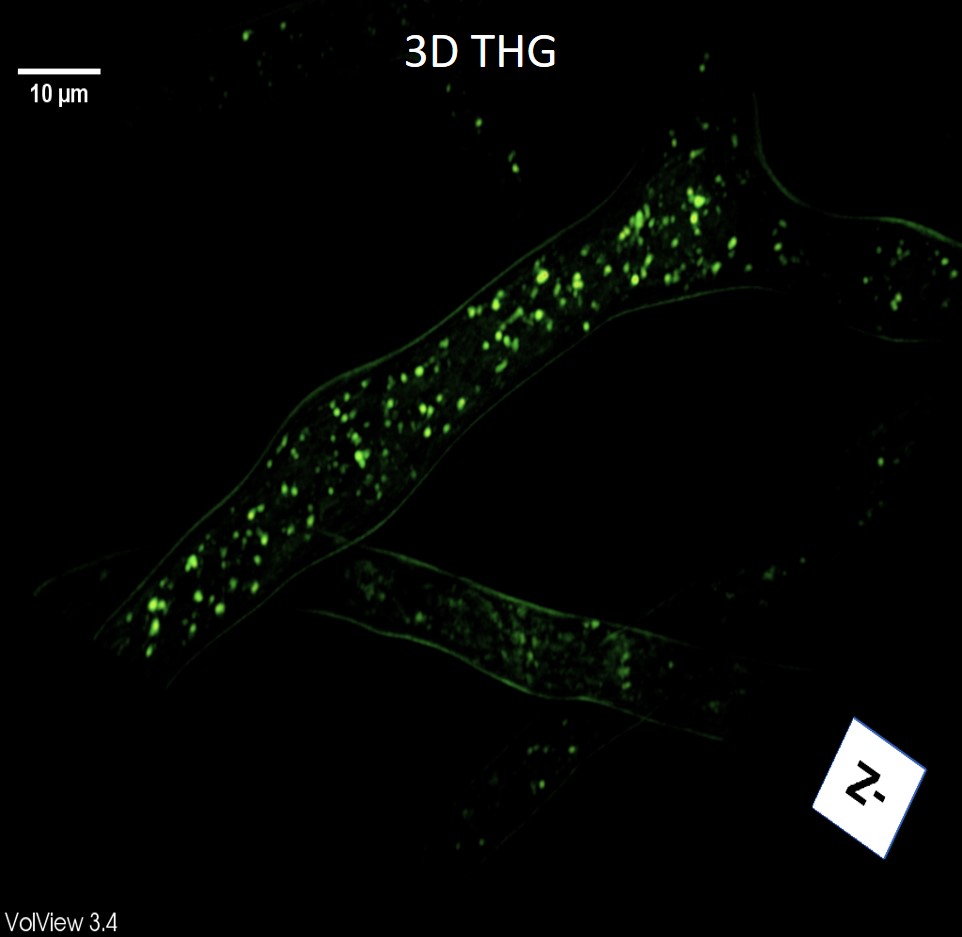
The Colocalization with Nile red dye confirmed that the round, bright structures on THG images, are lipid droplets. THG images showed a punctate, uniform distribution of lipid droplets in hyphae (Figure 1). In stressed cells, we obtained a higher number of LDs under nitrogen starvation. Lipid droplet diameters were measured in the THG images. The results show an increase in the number of middle-sized (0.4 - 0.7 μm) LDs after 3 hours of starvation, while the number of larger LDs remains the same or even smaller. After a longer starvation time, the increase of the LDs number in all size groups was evident. Also, we monitored the effect of sodium selenite (Na2SeO3) on lipid metabolism, i.e. the LDs number, size, and distribution.
Figure 1. 3D THG image of lipid droplets in hyphae of the fungus Phycomyces blakesleeanus
Although there is no lower limit on LD size [6], still, we could reliably perform physiological in vivo measurements of LD changes during nitrogen starvation and life cycle by our diffraction-limited imaging system. The structures in fungal hyphae detected by THG with a tightly focused laser beam correspond to LDs, based on colocalization experiments with Nile red.
In conclusion, the THG method can be used to label-free, in vivo, monitor LDs and their changes throughout the life of the fungus. [7]
- References
[1] Olzmann, J. A., & Carvalho, P. (2019, March 1). Nature Reviews Molecular Cell Biology, Vol. 20, pp. 137–155.
[2] Cabodevilla, A. G., Sánchez-Caballero, L., Nintou, E., Boiadjieva, V. G., Picatoste, F., Gubern, A., & Claro, E. (2013). Journal of Biological Chemistry, 288(39)
[3] Yelin, D., & Silberberg, Y. (2000). Molecular Crystals and Liquid Crystals Science and Technology Section B: Nonlinear Optics, 24(3), 267–270.
[4] Masters, B. R., So, P. T. C., & Mantulin, W. W. (2009). Journal of Biomedical Optics, 14(1), 019901.
[5] Débarre, D., Supatto, W., Pena, A. M., Fabre, A., Tordjmann, T., Combettes, L., Beaurepaire, E. (2006). Nature Methods, 3(1), 47–53.
[6] Long, A. P., Manneschmidt, A. K., Verbrugge, B., Dortch, M. R., Minkin, S. C., Prater, K. E., Dalhaimer, P. (2012). The Journal of Biological Chemistry, 13(5), 705–714.
[7] This work was supported by the Ministry of Education, Science, and Technological Development, Republic of Serbia