Phase and composition mapping of generation 3b high-energy-density LiNixCoyMnzO2 battery cathodes with transmission electron microscopy
- Abstract number
- 1453
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.1453
- Corresponding Email
- [email protected]
- Session
- PSA.4 - Batteries & Materials for Energy Conversion
- Authors
- C.F. Almeida Alves (1), E. F. Rauch (4), S. Nicolopoulos (3), Qiang Xie (2), Arumugam Manthiram (2), P. J. Ferreira (1, 2, 5)
- Affiliations
-
1. INL - International Iberian Nanotechnology Laboratory
2. Materials Science and Engineering Program, University of Texas at Austin
3. NanoMEGAS Sprl
4. SIMAP/GPM2 laboratory, CNRS-Grenoble INP
5. Mechanical Engineering Department and IDMEC, Instituto Superior Técnico, University of Lisbon
- Keywords
Layered NCM cathodes; Li-ion batteries; Precession Electron Diffraction; STEM;
- Abstract text
Summary:
The aim of this work is to understand the changes in phase and chemical distribution as a function of Ni content in polycrystalline high-energy-density NCM Generation 3b cathode materials.
Introduction
LiNixCoyMnzO2 (NCM) cathodes, which compose the current Generation 3a of LIB, offer higher specific capacity with operating voltage compared with LCO, in addition to lower cost and enhanced structural stability [1]. Yet, Li-based layered cathodes are susceptible to phase transformations during the charge/discharge processes [2], deteriorating the cycling performance [1]. For instance, it is reported that Li-rich NCM materials are composed by LiMO2/Li2MnO3 layers [1]. LiMO2, where M is a transition metal, is associated with a trigonal (R-3m) phase, whereas Li2MnO3 is associated with a monoclinic (C2/m) phase [3]. These layered materials still exhibit a large degree of cation disorder and hence, Ni2+ may exchange with Li+ in the Li-layer, leading to interlayer mixing, which disrupts the Li+ pathways and creates a continuous MO2 layer, lowering the Li+ mobility [4]. In the near future, the Generation 3a of commercial LIBs cathodes, composed by porous micrometer polycrystalline particles exhibiting a mixture of Mn-rich and Ni-rich phases, will evolve into Generation 3b cathodes, with high Ni content and consequently high-energy-density.
Materials and Methods
The chemical composition and structure of porous micrometer polycrystalline particles with Ni compositions ranging from 0.70 up to 0.90 wt. % were investigated using FIB-SEM, double-corrected TEM-STEM, EDS mapping and precession electron diffraction. Cross-section samples were prepared using a Helios 450S (FEI) focused ion beam. High angular annular dark field (HAADF) STEM images were acquired in a double-corrected FEI Titan microscope (equipped with a Schottky FEG gun) operated at 200 keV. The images were recorded using a convergence angle of 21 mrad. A camera length of 115 mm was selected, which collects electrons between 56 and 200 mrad. Energy dispersive X-ray spectroscopy mapping (EDS-mapping) was performed in the same microscope, equipped with a Super-X EDS detector, to record iterative maps. Precession electron diffraction maps were acquired in a JEOL JEM 2100F microscope (equipped with a FEG gun) operated at 200 keV in nano-probe mode with a nominal probe size of approximately 2 nm and step size of 5 and 10 nm.
Results and discussion
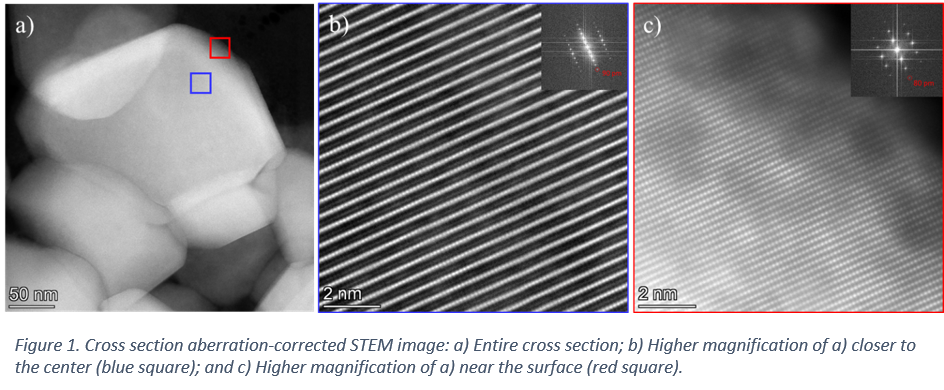
Particles with a Ni content ranging between 0.70 and 0.90 wt. % of Ni were analysed. Slice-and-view SEM/FIB analysis revealed a geode-like morphology, independently of the composition. Yet, as the Ni content increases, the particle porosity decreases. Furthermore, precession electron diffraction coupled with STEM-EDS revealed differences in structure across a single particle, which are concomitant with variations in chemical composition, in particular Ni content. In fact, atomic-resolution STEM images (Fig. 1) are in good agreement with the precession electron diffraction results, which reveal variations in orientation within a single crystalline particle from the particle centre (Fig. 1 b) to the surface (Fig. 1 c).
Conclusion
The results have shown a predominance of the trigonal (R-3m) phase for all the Ni contents studied despite the fact that an increase of Ni leads to segregations of Mn.
- References
References
[1] N. Nitta, F. Wu, J.T. Lee, G. Yushin, Li-ion battery materials: present and future, Mater. Today. 18 (2015) 252–264.
[2] H. Zhou, Two-phase transition of Li-intercalation compounds in Li-ion batteries, Mater. Today. 17 (2014) 451–463.
[3] K.A. Jarvis, Z. Deng, L.F. Allard, A. Manthiram, P.J. Ferreira, Atomic structure of a lithium-rich layered oxide material for lithium-ion batteries: evidence of a solid solution, Chem. Mater. 23 (2011) 3614–3621.
[4] S.B. Schougaard, J. Bréger, M. Jiang, C.P. Grey, J.B. Goodenough, LiNi0. 5+ δMn0. 5–δO2—A High‐Rate, High‐Capacity Cathode for Lithium Rechargeable Batteries, Adv. Mater. 18 (2006) 905–909.
[5] Acknowledgements: The authors would like to acknowledge that this project has received funding from the EU Framework Programme for Research and Innovation H2020, scheme COFUND – Co-funding of Regional, National and International Programmes, under Grant Agreement 713640.